Stokes radius
The Stokes radius, Stokes-Einstein radius, or hydrodynamic radius RH, named after George Gabriel Stokes, is not the effective radius of a hydrated molecule in solution as often mentioned. Rather it is the radius of a hard sphere that diffuses at the same rate as the molecule. The behavior of this sphere includes hydration and shape effects. Since most molecules are not perfectly spherical, the Stokes radius is smaller than the effective radius (or the rotational radius). A more extended molecule will have a larger Stokes' radius compared to a more compact molecule of the same molecular weight.
In liquids where there are considerable interactions between solute and solvent molecule, the Stokes' radius (of a perfect sphere) is proportional to frictional coefficient f and inverse proportional to viscosity η as follows:
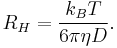
where, 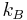 is the Boltzmann constant (in J K−1),
is the Boltzmann constant (in J K−1), 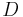 is the diffusion coefficient (in m2s−1) and
is the diffusion coefficient (in m2s−1) and 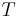 is the temperature in kelvins. The frictional coefficient is determined by the size and shape of the molecule under consideration.
is the temperature in kelvins. The frictional coefficient is determined by the size and shape of the molecule under consideration.